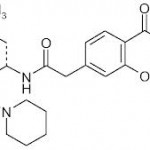
REPAGLINIDE
The first of a new class of drugs for treating Type 2 diabetes. Cleared for marketing by the U.S. Food and Drug Administration in April 1998, repaglinide (brand name Prandin) is the first of a new class of oral diabetes drugs called meglitinides. It follows on the heels of a variety of diabetes drugs recently introduced, including acarbose (Precose) and metformin (Glucophage).
Like the sulfonylurea drugs (which include glyburide, glimepiride, and glipizide), repaglinide works by stimulating the beta cells of the pancreas to release insulin. Yet, unlike the sulfonylureas,
repaglinide is cleared very quickly from the bloodstream. It also stimulates the release of insulin in a glucose-dependent fashion: The more glucose there is in the bloodstream, the more insulin the pancreas secretes. These aspects make repaglinide especially suitable for controlling the surges in blood glucose that follow meals.
Studies have shown that repaglinide is as effective as glyburide and more effective than glipizide in controlling blood sugar levels. It also appears that repaglinide works well in combination with metformin: Studies have shown that a combination of these two drugs controls blood sugar levels more effectively than either drug used alone.
The frequency of side effects of repaglinide appear to be similar to that of the sulfonylureas. The most common side effects include hypoglycemia (low blood sugar) and mild weight gain. The most serious drawback to repaglinide appears to be that people may find it inconvenient to take one dose with each meal as recommended.